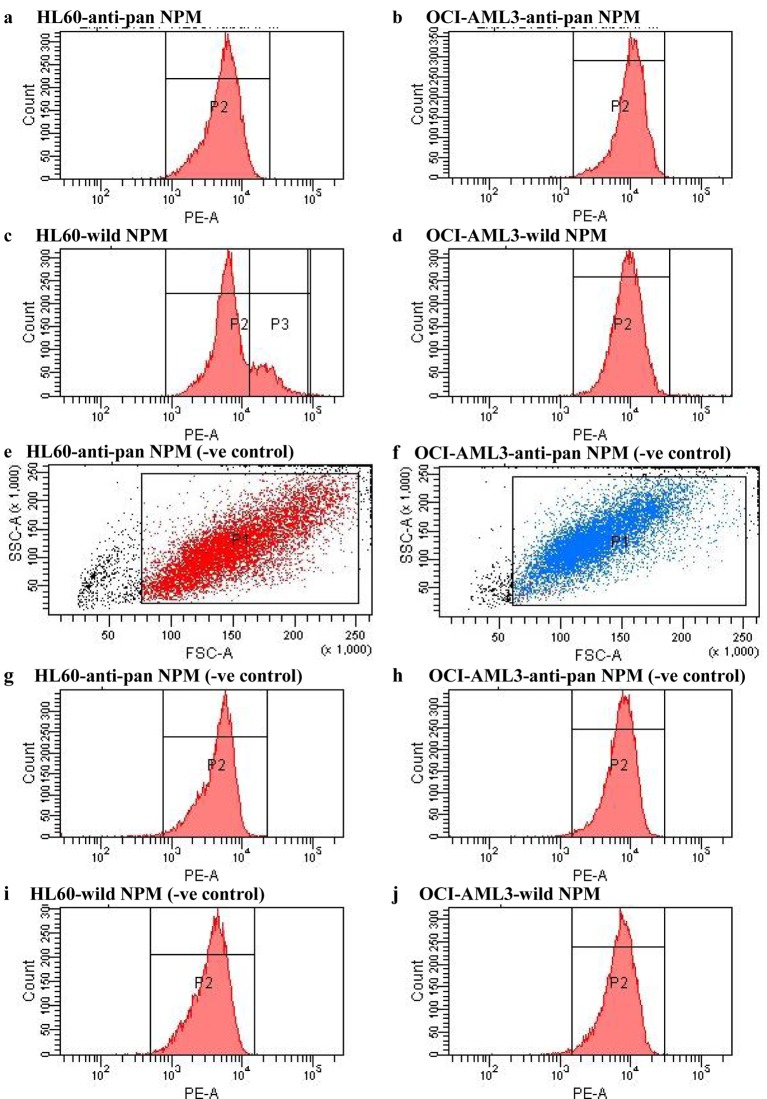
In our earlier work, we provided sturdy proof that nucleophosmin (NPM) gene mutation has an very important place in leukemogenesis of main acute myeloid leukemia (AML). Furthermore, we speculated a model new centered treatment in victims with main AML and bearing mutated NPM (mNPM).
Based on these outcomes together with findings of completely different researchers, it was necessary to develop a way for proper detection of mNPM. Our methodology based on utilizing the most recent transfer cytometeric strategies and gadgets in measuring mNPM. Attributed to their availability and technical feasibility, we used human leukemia cell traces to validate our methodology.
The necessary findings had been differential expression of wild-type NPM (wtNPM) all through the same sample. Furthermore transfer cytometry (FCM) was a straightforward straightforward instrument for quantitative assay of mNPM.
In this work we developed an trendy method that may enable quantitative assay of mNPM, and ease its use as a biomarker in cytogenetic and molecular prognostication of main AML. In addition the look at immediate that FCM would possibly differentiate mNPM expression inside cells of the similar affected particular person thus could be used for monitoring of minimal residual sickness.
Accuracy of a reverse dot blot hybridization assay for simultaneous detection of the resistance of Four anti-tuberculosis medication in Mycobacterium tuberculosis isolated from China.
Drug resistant tuberculosis poses a tremendous downside for tuberculosis administration worldwide. Timely dedication of drug resistance and environment friendly explicit particular person remedy are necessary for blocking the transmission of drug resistant Mycobacterium tuberculosis. We aimed to find out and think about the accuracy of a reverse dot blot hybridization (RDBH) assay to concurrently detect the resistance of Four anti-tuberculosis medication in M. tuberculosis isolated in China.
In this look at, we utilized a RDBH assay to concurrently detect the resistance of rifampicin (RIF), isoniazid (INH), streptomycin (SM) and ethambutol (EMB) in 320 medical M. tuberculosis isolates and in distinction the outcomes to that from phenotypic drug susceptibility testing (DST) and sequencing.
The RDBH assay was designed to verify as a lot as 42 samples at a time. Pearson’s chi-square check out was used to compute the statistical measures of the RDBH assay using the phenotypic DST or sequencing as a result of the gold commonplace methodology, and Kappa id check out was used to seek out out the consistency between the RDBH assay and the phenotypic DST or sequencing.
The outcomes confirmed that the concordances between phenotypic DST and RDBH assay had been 95% for RIF, 92.8% for INH, 84.7% for SM, 77.2% for EMB and the concordances between sequencing and RDBH assay had been 97.8% for RIF, 98.8% for INH, 99.1% for SM, 93.4% for EMB.
Compared to the phenotypic DST outcomes, the sensitivity and specificity of the RDBH assay for resistance detection had been 92.Four and 98.5% for RIF, 90.Three and 97.3% for INH, 77.Four and 91.5% for SM, 61.Four and 85.7% for EMB, respectively; in comparability with sequencing, the sensitivity and specificity of the RDBH assay had been 97.7 and 97.9% for RIF, 97.9 and 100.0% for INH, 97.Eight and 100.0% for SM, 82.6 and 99.1% for EMB, respectively.
The turnaround time of the RDBH assay was 7 h for testing 42 samples.Our information immediate that the RDBH assay would possibly perform a speedy and atmosphere pleasant methodology for testing the resistance of M. tuberculosis in the direction of RIF, INH, SM and EMB, enabling early administration of acceptable remedy regimens to the affected drug resistant tuberculosis victims.