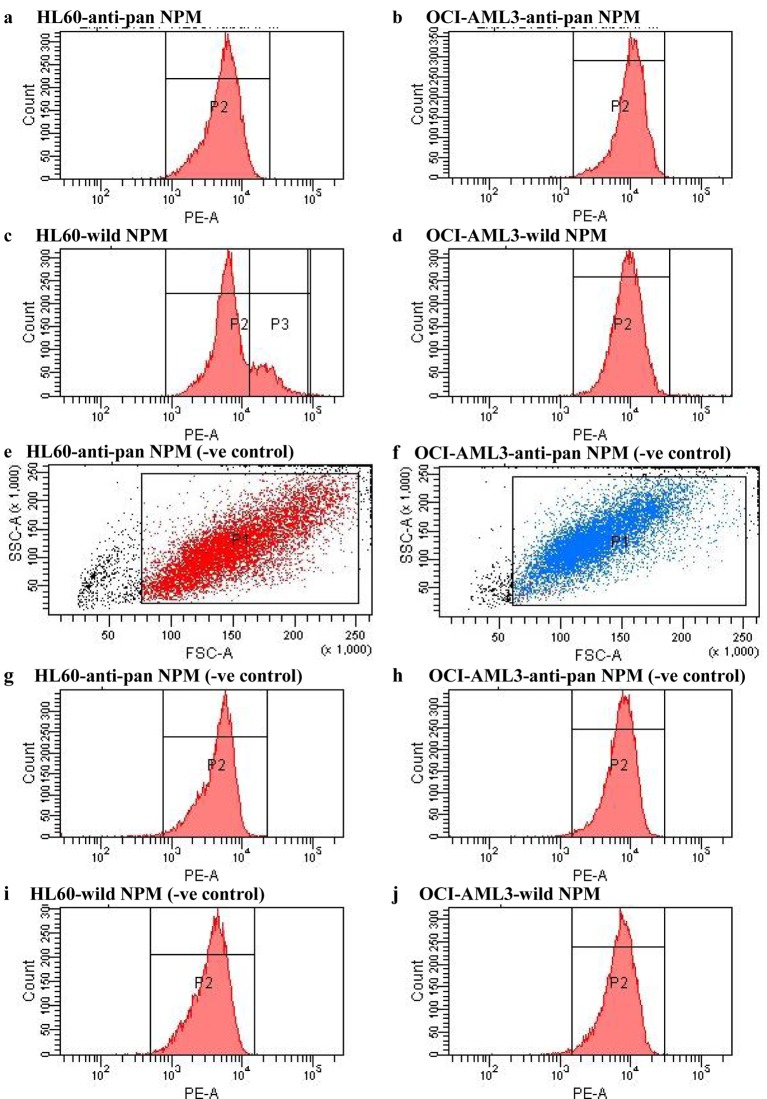
Autochthonous leishmaniasis attributable to Leishmania martiniquensis circumstances in Thailand have dramatically elevated within the latest years. L. martiniquensis an infection primarily happens in immunocompromised sufferers, particularly AIDS sufferers.
In Thailand, amphotericin B is the one drug obtainable for leishmaniasis remedy, and a few sufferers relapse after amphotericin B remedy. Moreover, the efficacy of anti-leishmanial drugs against L. martiniquensis has not been evaluated so far. In this examine, we decided the efficacy of numerous anti-leishmanial drugs against the promastigote and intracellular amastigote phases of L. martiniquensis using a colorimetric assay.
Two strains (CU1 and CU1R1) have been remoted from leishmaniasis HIV co-infected affected person from Songkhla province, southern Thailand. The CU1 pressure was remoted from the affected person in 2011, and CU1R1 was remoted from the identical affected person in 2013, when he was recognized as relapse leishmaniasis.
The third pressure (LSCM1) used on this examine has been remoted from immunocompetent affected person from Lamphun province, northern Thailand. All strains have been recognized as L. martiniquensis by sequencing of ribosomal RNA ITS-1 and enormous subunit of RNA polymerase II gene.
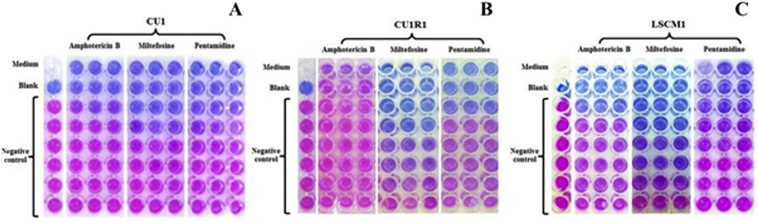
Bioassays have been performed each with promastigote and intracellular amastigote phases of the parasite. All L. martiniquensis strains have been examined against amphotericin B, miltefosine and pentamidine to decide the efficacy of the drugs against the parasite by using a PrestoBlue.
The efficacy of miltefosine and pentamidine exhibit no vital distinction between every stage of L. martiniquensis amongst all strains. Surprisingly, the promastigote and intracellular amastigote of the CU1R1 isolate, which was remoted from a relapsed affected person after amphotericin B remedy, exhibited a two-fold elevated inhibitory focus (IC50) against amphotericin B in contrast with different strains, and the distinction was statistically vital (p < 0.05).
Moreover, intracellular amastigotes remoted from CU1R1 exhibited barely elevated susceptibility to amphotericin B in contrast with the promastigote (p < 0.05).
The consequence of this experiment is a scientific evident to help that in case of relapsed leishmaniasis attributable to L. martiniquensis, rising dosage of amphotericin B is crucial.
Moreover, this examine additionally decided efficacy of different anti-leishmanial drugs for remedy the leishmaniasis in Thailand in case of these drugs can be found within the nation and the clinicians ought to have various drugs for remedy leishmaniasis in Thailand other than amphotericin B.
A novel circulate cytometry-based assay to measure compromised B cell receptor signaling as a prognostic think about persistent lymphocytic leukemia.
Chronic lymphocytic leukemia (CLL) is the commonest leukemia in adults. In the previous years, new therapeutic approaches (e.g., ibrutinib or venetoclax) have been established and significantly improved remedy of CLL. However, full management or remedy of the illness haven’t been reached to date.
Thus, dependable prognostic markers are an crucial for remedy choices. Recent research have revealed a vital position for B cell receptor (BCR) signaling within the pathogenesis, prognosis, and remedy of CLL.
A heterogeneous response to receptor stimulation with anti-IgM remedy culminating in numerous calcium flux capabilities has been demonstrated by a number of authors. However, the strategies employed haven’t reached scientific software.
Here, we report on a circulate cytometry-based assay to judge calcium flux capabilities in CLL and display that compromised BCR signaling with diminished calcium flux is related to a considerably higher scientific end result and development free survival. In abstract, our knowledge strongly help the position of compromised BCR signaling as an necessary prognostic marker in CLL and establish a novel diagnostic device for its evaluation in scientific settings.